We provide the following engineering services within our core technological fields: powder technology, biotechnology and precision cleaning technology. We assemble our engineering teams in project basis in order to design and produce processes that take into account not only the customer’s requirements, but also safety, ease of maintenance, and operation costs. Our global partner network allows us to deliver products and services under our consistent quality control systems all over the world.
Project management



In EPC or EPIC project, we undertake an entire project or partially within our own technological fields. In addition to delivering our standard products, if our products are the core of a project, we will be involved from the initial consideration stage of the customer’s project planning. Even after product delivery, we keep supporting our customers for their entire project lifecycles, including operation and maintenance management.
Flow example of our standard product delivery
- Basic design, process design
- Design review
- Detailed design
- Manufacturing, procurement, assembly, test run
- FAT (Factory Acceptance Test)
- Delivery, installation, utility connections
- Test run, acceptance inspection
- Operation and maintenance services
Flow example of EPC/EPIC projects
- Feasibility study support
- Conceptual design
- FEED support
- Basic design, process design
- Design review
- Detailed design
- Manufacturing, procurement, temporary assembly, test run
- FAT (Factory Acceptance Test)
- Delivery, assembly, construction, installation, utility connections
- Test run
- SAT (Site Acceptance Test), acceptance inspection
- Operation and maintenance services
Design & Engineering



From small equipment for research and development to large-scale manufacturing processes, we provide unique products based on our advanced technology background. In addition to standard products, customization is always available according to individual requirements. We design products that take into account the unique constraints of capabilities and environmental conditions at the installation environment, and provide products and processes from the user’s perspective.
Design



We propose the most suitable products based on the basic requirements of our customers. If the product is available with standard specifications, we will present specifications, outline drawings, and related materials after confirming that the specifications meet the customer’s requirements. Even in the case of standard specifications, it is possible to change the power supply, voltage, and utility standards depending on the country or region where the product is carried out. If our process is the core of EPC or EPIC project, we may be involved from the feasibility study or overall conceptual design.
Conceptual design
We perform the necessary calculations and validation to create an optimal process. Calculations include thermodynamics, mass balance, fluid simulation, process simulation, etc. as necessary. We also conduct trials and analytical measurements at our Powder Technical Center, Biotechnical Center, and Cleaning Test Center to reflect actual evaluations and improve design accuracy.
Basic design & Process design
We start with P&ID design, which serves as a guidepost for the process, and determine the equipment configuration and required capacities. We design the most suitable layout for the given environmental conditions, taking into consideration the space limitations of the installation location, ease of maintenance, energy efficiency, etc. We also carry out electrical control design, including power and control boxes, in parallel.
Design review
In addition to required capacities and functions, we comprehensively evaluate safety risk assessment, operability, productivity, environmental compatibility, regulatory compliance, and maintainability. We identify points of concern or unclear items, and if there are any problems, we re-consider for an appropriate improvement.
Detailed design
After passing the design review, we start a detailed design process and complete detailed manufacturing drawings, bill of materials, and electrical/instrumentation designs.
Production & Procurement


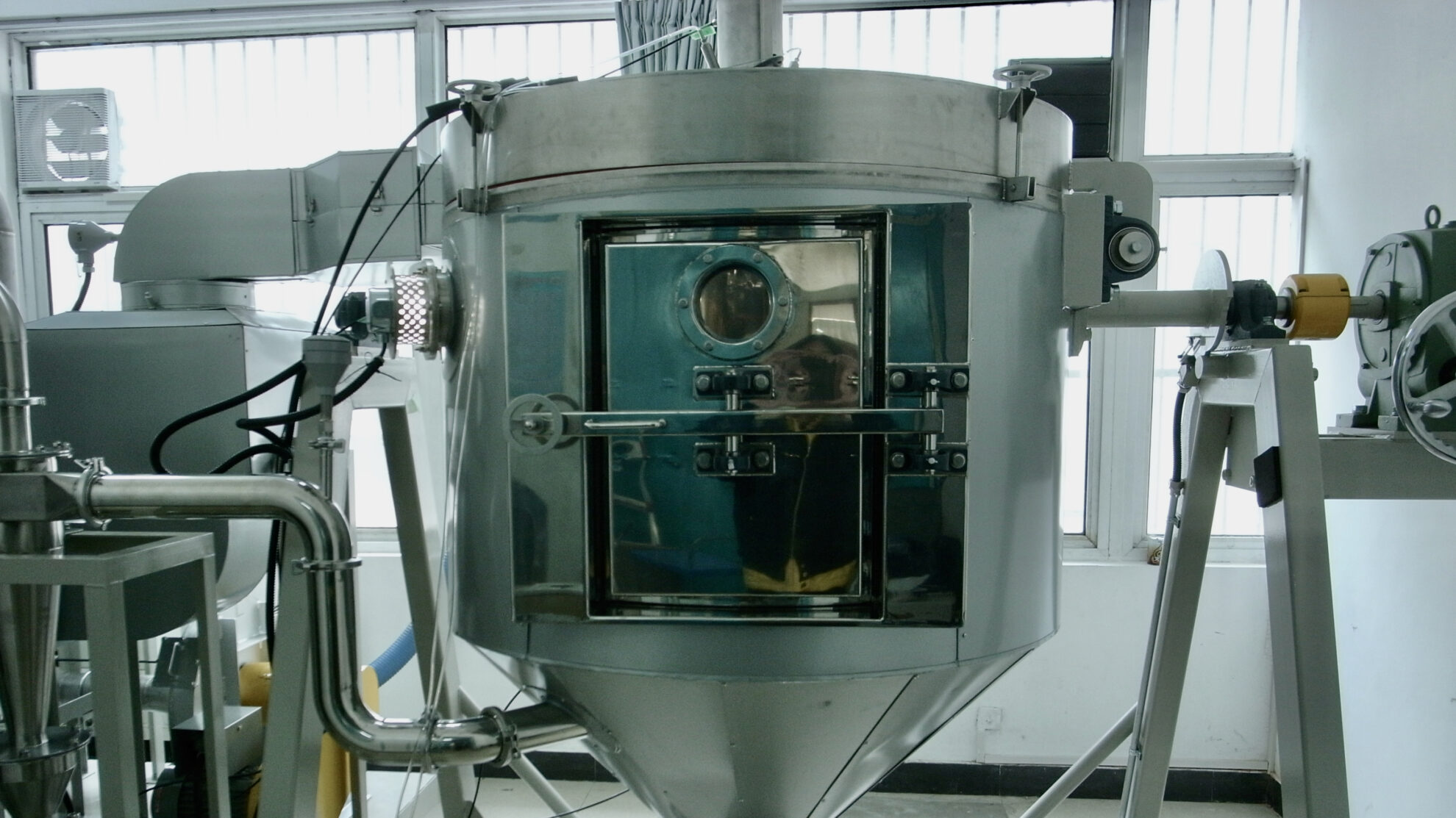
We procure fabricated products, components and parts based on manufacturing drawings and BOM. After placing orders, we manage the delivery schedule and quality control of the procured items, and then deliver them to the assembly location or directly transport to the site. We source from our global network after comprehensively comparing quality, delivery, and price.
Assembly



Some of our standardized products are assembled at our own assembly center. We perform assembly, wiring, and test run, and then pass the quality inspection and FAT before shipping. If on-site re-assembly or construction is required, we select the optimal assembly location from our network with the consideration of the product type and geographical conditions. In order to reduce the workload for on-site construction, we carefully plan the division of manufactured products and module products at the time of shipment. These items are shipped to the site after passing through our internal quality inspection and FAT.
On-site delivery & Construction



Based on the construction plan, we carry out our construction work by taking quality and safety aspects into account and securing appropriate resources. We precisely manage every single part of the products and materials from welding, polishing, heat insulation, painting, piping, electrical wiring, utility connections, etc., to the installation of modular products and instrumentation, and the assembly and installation of the entire process. We consider safety management to be the most important responsibility in construction phase. In addition to complying with on-site safety standards, we additionally provide education and guidance based on our own standards to our contractors and suppliers. After completion of the construction phase, we move to quality inspection and followed by a test run operation.
Test run & Commissioning



After completion of installation, we start I/O, functional inspection of each component, software verification and interlocks confirmation. We start test run and commissioning after completing all of the on-site inspection processes. During commissioning, we verify that all functions and performance in the process are met in accordance with the design specifications and URS. Commissioning requires experience and expertise in a wide range of fields. Not only knowledge of the process and operational controls, but also analytical ability such as physical and chemical knowledge of the products and raw materials are required in order to confirm that the output as a result of the process meets the customer’s quality standards. SAT is also conducted in parallel with the commissioning. After final confirmation that the process capacity and safety controls such as interlocks, as well as safety rules and environmental regulations are met, the process is officially accepted.
Quality control


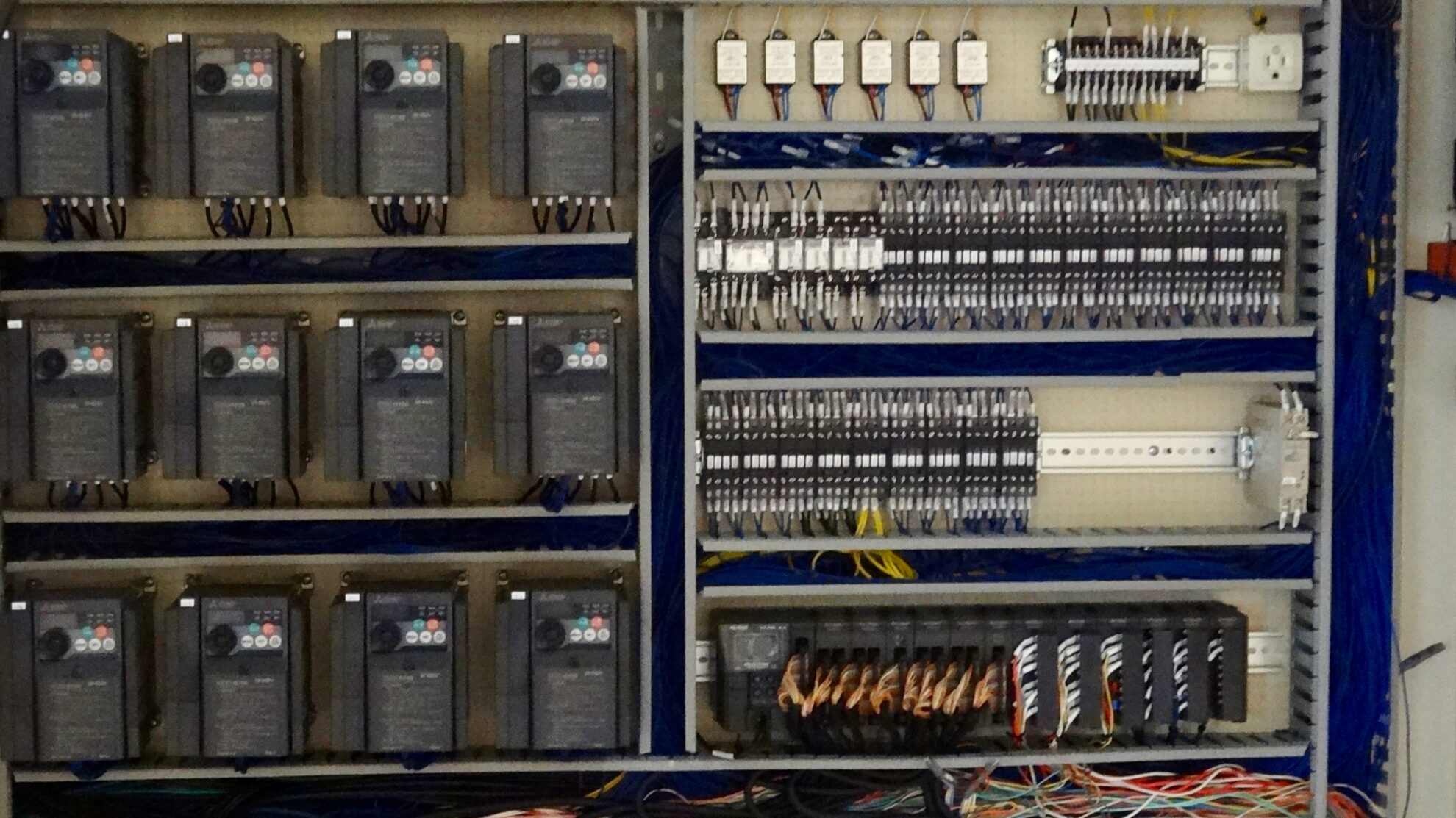
We carry out projects with our integrated quality control system, from design phase to delivery. We strictly enforce our own quality policy and quality control systems. Regarding medical devices, we manufacture products under a legally compliant QMS system.
QC policy
In order to provide products and services that contribute to customer value, we comply with customer requirements and quality control system requirements, strive to maintain their effectiveness, and constantly pursue improvements in customer satisfaction.
QMS policy (Medical equipment)
In order to provide excellent quality medical equipment, we comply with the requirements of the quality management system, strive to maintain its effectiveness, and constantly pursue improvements in customer satisfaction.
If validation in compliance with GLP or GMP is required in pharmaceutical industry, we provide appropriate support as necessary. We provide necessary support for verification and documentation at each stage of DQ (Design Qualification), IQ (Installation Qualification), OQ (Operating Qualification), and PQ (Performance Qualification). We support a verification process including design-related documents such as FS (Functional Specifications), P&ID, and various drawings, material certificates and calibration certificates for instruments and equipment at IQ, OQ, and PQ. Regarding CSV (Computerized System Validation), we propose items that can be supported depending on the software and control system in the process, as well as the corresponding category classification. *This service may not be available for some products and processes.
Operation & Maintenance



After hand-off, the process must operate safely and stably. Appropriate maintenance and maintenance plans are required while maintaining high productivity under a year-round operation plan. The most important factor in O&M is that the operations and maintenance team has the appropriate knowledge. Even after handing off the process, we continue to share the operational status with our customers and provide regular training as necessary. For example, if there is a major change in personnel in the operations or maintenance team, technical training sessions may be provided again.
For maintenance inspections and periodic repair works, we propose an appropriate plan based on the annual operation plan. In addition to maintenance management, we also support regular calibration or validation works. If a sudden problem occurs, we will quickly dispatch our engineering team in order to minimize the impact on the operation plan. Overseas, where response speed possibly becomes slow, we work with our local partners to verify the problem online, and then have the partner conduct an on-site inspection and take appropriate actions.
We also support future planning of process renewal and modifications. We propose plans that contribute to increasing the value of the processes, such as improving process productivity and energy efficiency. It is possible to improve each component or partially add our latest technologies. We also have many achievements in updating aging processes by reusing usable equipment. We support productivity and quality improvement as well. If there is a concern that productivity or quality may deteriorate due to changes in operating conditions, recipes or raw materials, we review the design or operating conditions again and identify any necessary adjustments.
Case study
Spray dryer (Japan)



One of our loyal customers, a collagen peptide manufacturer, has continued to increase its spray drying production capacity to meet growing demand since its first spray dryer installation tens of years ago. From small scale lab spray dryers to multiple large production plants, the customer has always selected our spray dryers until now. The customer has expanded its spray drying capacity together with applying our updated new technologies in its process year by year. The adoption of new technologies are not only applied to new plants, but also to its existing plants, enabling continuous improvements of production efficiency and productivity.
For the customer’s R&D, in addition to its in-house operation, the customer also collaborates with our Powder Technical Center to increase its research efficiency. In daily research, we contribute to our customer’s development team by providing advice on operating conditions, etc. We also supplements our analytical measurement facilities that the customers do not own, making it possible to evaluate the products in multiple ways. Through our long-term strong partnership with the customer, we contribute to increasing customer value not only by installing our processes, but also by improving production capacity and efficiency, and supporting their R&D.
* Images may differ from the actual project and may be used for illustrative purposes only.
Incubator shaker (USA)

Our customer, an American pharmaceutical company, develops drugs by combining its drug delivery system (DDS) technology with approved active pharmaceutical ingredients (APIs), and has a rich technology portfolio in liposomes. In 2019, the customer found our technology through an introduction from its supplier and we received their inquiry thanks to the introduction. They searched a possible shaking technology that could operate at high speed to handle the number of vials required for their product preparation process all over the world, but they were unable to find a suitable manufacturer. After their contact to us, we started reviewing their requirements. The technical standard required by the customer was very high: 5,000 vials per batch (total weight 200 kg) were planned to be run at 400 rpm for 24 hour/10 day cycle continuous operations. After conducting our in-house technical review, we determined that it was possible to meet the requirements, and the project officially began.
After basic design, calculations, safety risk assessment, environmental compatibility verification, and regulatory compliance, we proceeded to the detailed design and manufacturing process. Since the beginning of 2020, the spread of the COVID-19 has forced our project progress into a difficult situation. Although there were some problems such as delays in parts delivery, the product was successfully completed. However, due to the travel restrictions worldwide during the pandemic, it became impossible to conduct on-site commissioning. As a result of the discussion with the customer, we conducted remote FAT, IQ, OQ, and SAT online, and successfully passed the acceptance inspection. After the delivery, the customer has successfully moved to their commercial operation as planned and continues to maintain stable production even under high-capacity, high-speed rotational conditions.
* Images may differ from the actual project and may be used for illustrative purposes only.
Precision cleaning system (Japan, Malaysia)



Our customer, a Japanese industrial equipment manufacturer, is a global company with multiple products that have top market shares in the world. The customer was planning to simultaneously launch new product production lines at its domestic mother factory and Malaysian factory. The project was planned to use the same equipment for all manufacturing processes in order to maintain the same level of quality on the manufacturing lines. The customer was looking for a supplier that could launch the two cleaning processes at the same time with the same specifications both in Japan and Malaysia. There are many cleaning systems suppliers in Japan, but many suppliers produce their products domestically and export them outside of Japan. Additionally, the customer specified to use many of domestic parts in the process, and the process requirements for the Malaysian factory were the same. On the other hand, the customer was also looking for a supplier that could manufacture the cleaning system in Malaysia for a possible reduction of the system price and transportation costs.
Through our partnership with JKS ENGINEERING, a Malaysian cleaning process manufacturer, we are able to manufacture the processes in Malaysia. The customer was able to conduct a cleaning trial and evaluate the performance using both our cleaning test center in Japan and the testing facility in Malaysia. After the project was launched, specifications, P&ID, and detailed designs were completed in Japan, and process for the domestic mother factory were manufactured domestically, while another process for the Malaysian factory was manufactured locally in Malaysia. We have also reduced our customers’ total investment costs by reducing export and transportation costs. JKS ENGINEERING provides on-site maintenance services for the cleaning system installed in Malaysia so that the speed of O&M response has also been praised.
* Images may differ from the actual project and may be used for illustrative purposes only.
Platelet shaker (Japan)

We manufacture and supply platelet shakers to Japanese Red Cross Society and hospitals. Platelet products, which are blood products for transfusion, are concentrated blood platelets that have a hemostasis function. Platelet products are used to replenish platelets to stop bleeding or prevent bleeding when there is a decrease in platelet count and/or decreased function. Platelet products deteriorate more quickly during storage than red blood cells. Currently, in Japan, platelet products are stored at the temperature range of 22+/-2℃ with continuous shaking operation. If products are stored in a low-temperature environment such as refrigeration, there are problems such as a reduction in the in-vivo lifespan after transfusion, and changes in platelet morphology that reduce the effectiveness of transfusion. For shaking operation, products are stored on trays with constant reciprocal shaking operation at 60 rpm. If products are kept in a static state, platelet aggregation ability will decrease as the pH decreases. Therefore, platelet products should be used as soon as possible after taking them out of the platelet shaker. In order to stably supply platelet products under safe and consistent quality, it is essential to use reliable platelet shakers that can constantly operate and store them uniformly within a certain temperature range. In addition, platelet products rapidly deteriorate in quality due to a decline in platelet aggregation ability in a static state, so platelet storage shakers must be robust enough to operate without failure.
Our platelet shakers boasts unrivaled robustness and stability, thanks to the shaking technology accumulated over many years in the pharmaceutical and food industries. A unique drive system that slides the stand on four shafts allows it to maintain low noise and high durability over a long period of time. Additionally, in the event of an unexpected power outage or input power failure, the product is equipped with an uninterruptible power supply as standard, which will issue a power outage alarm and record temperatures for a certain period of time. Our products have been delivered to many of Japanese Red Cross Society’s blood centers nationwide as well as large hospitals, thanks to the long-standing reputation for product stability and reliable maintenance services including calibration and validation. We have a proven track record of platelet shakers. By properly maintaining the shakers operating throughout Japan, we will continue to contribute to the stable supply of platelet products in Japan.
* Images may differ from the actual project and may be used for illustrative purposes only.
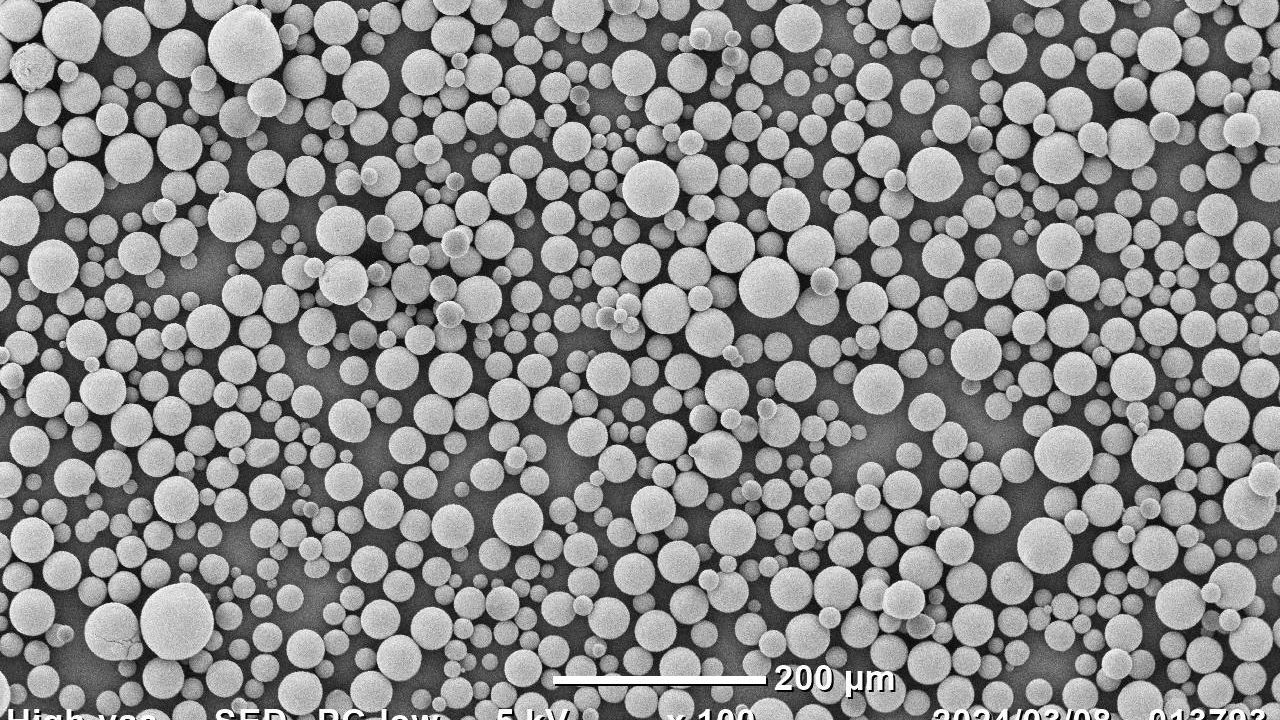
Freeze Granulator (Japan)


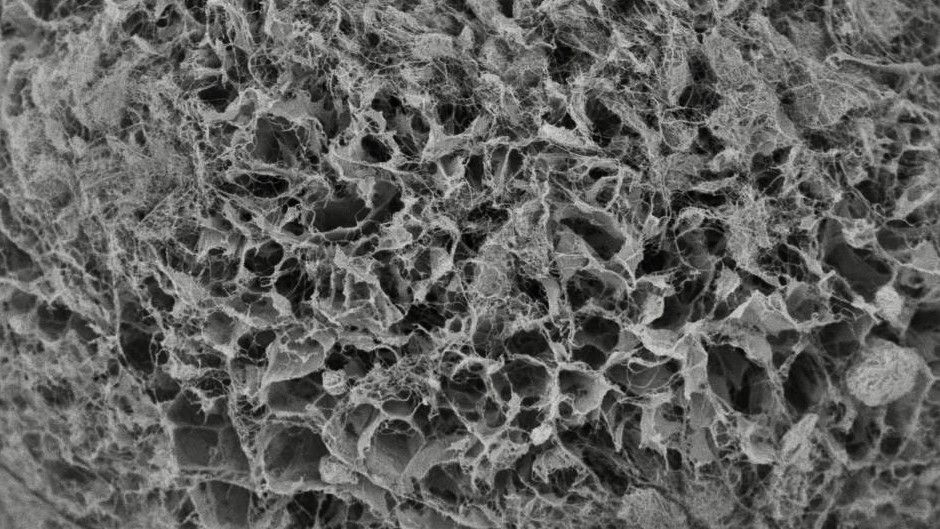
We began our in-house research and development with the aim of scaling up the freeze granulation process, which previously only existed as a simple unit for experiments, and after about four years of development, we achieved to develop the world’s first freeze granulator CS30 (patented) for mass production using a dry cooling method in 2019.
In 2020, we were awarded jointly with the National Institute of Advanced Industrial Science and Technology (AIST), Yokohama National University, and Yokohama TLO for the FY2020 Strategic Fundamental Technology Advancement Support Project by the Ministry of Economy, Trade and Industry (Project name: Research and Development of Spray Freeze Granulation Drying Process for Advanced Fine Ceramics), and proceeded with the development of further advanced freeze granulators. In 2023, we developed our latest generation freeze granulator CS220 (patented). CS220 is “liquid nitrogen-free” dry cooling freeze granulation process using our original high-efficiency cooling system. As the process does not use liquid nitrogen, it has advantages in safety, environment, installation costs, and operating costs.
Granules obtained by freeze granulation are spherical in shape, have excellent fluidity, and can maintain low density and high homogeneity. The particle size range of the granules is relatively wide, ranging from 10 to 500 µm, and the tap density equivalent to or higher than spray drying granules can be obtained. Freeze granulation is a new mass production powder manufacturing process that we succeeded in developing for the first time in the world. Therefore, there are not many actual application cases yet. The quality of applications currently manufactured using spray dryers can be improved by using freeze granulators. Compared to spray drying, granules are not affected by heat and can be low-density, homogeneous and well structured, so it is expected to contribute to product quality improvement and material development in a wide range of industrial fields in the future.
* Images may differ from the actual project and may be used for illustrative purposes only.